Research
Sonogenetics
What is Sonogenetics? Sonogenetics uses ultrasound to non-invasively manipulate neurons and other cells expressing exogenous protein channels.
Current Sonochannels. Our lab is focused on the identifying and optimizing ultrasound-sensitive channels. While our early work using TRP-4 in C. elegans established this field, we have since expanded our toolkit to target mammalian systems and diverse cellular functions. We recently established human TRPA1 (hsTRPA1) as a highly effective, exogenous sonochannel for non-invasive cellular modulation.
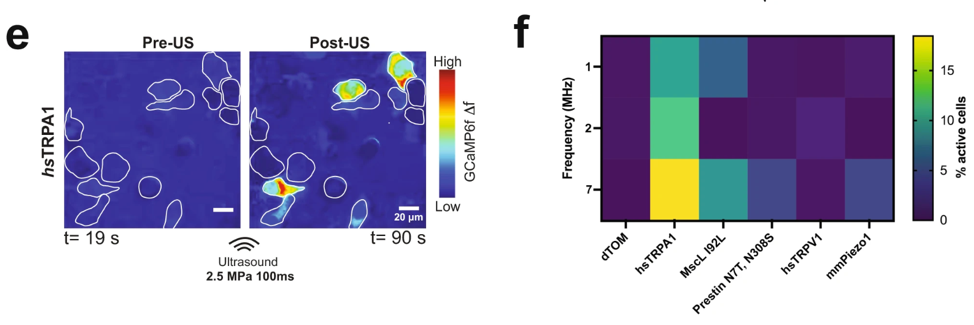
- Sensitivity to ultrasound: We demonstrated that the overexpression of hsTRPA1 renders mammalian cells, including HEK293T and primary neurons, sensitive to low-intensity ultrasound.
- Specific and Robust Activation: Ultrasound stimulation of hsTRPA1-expressing cells resulted in rapid calcium influx demonstrating that the channel can drive downstream signaling pathways.
- Safety and Inertness: We are committed to using ultrasound parameters that do not cause significant heating or cytotoxicity; cells lacking our sonochannels remain unresponsive, ensuring specificity for targeted modulation.
Building on this foundation, we are now generating mutants to achieve enhanced sensitivity to ultrasound, faster kinetics, and optimized expression in diverse cell types. Beyond activation, we are also actively developing inhibitory Sonochannels. These channels will allow for the non-invasive silencing of cellular activity, providing a non-invasive “on and off” switch for biological circuits.
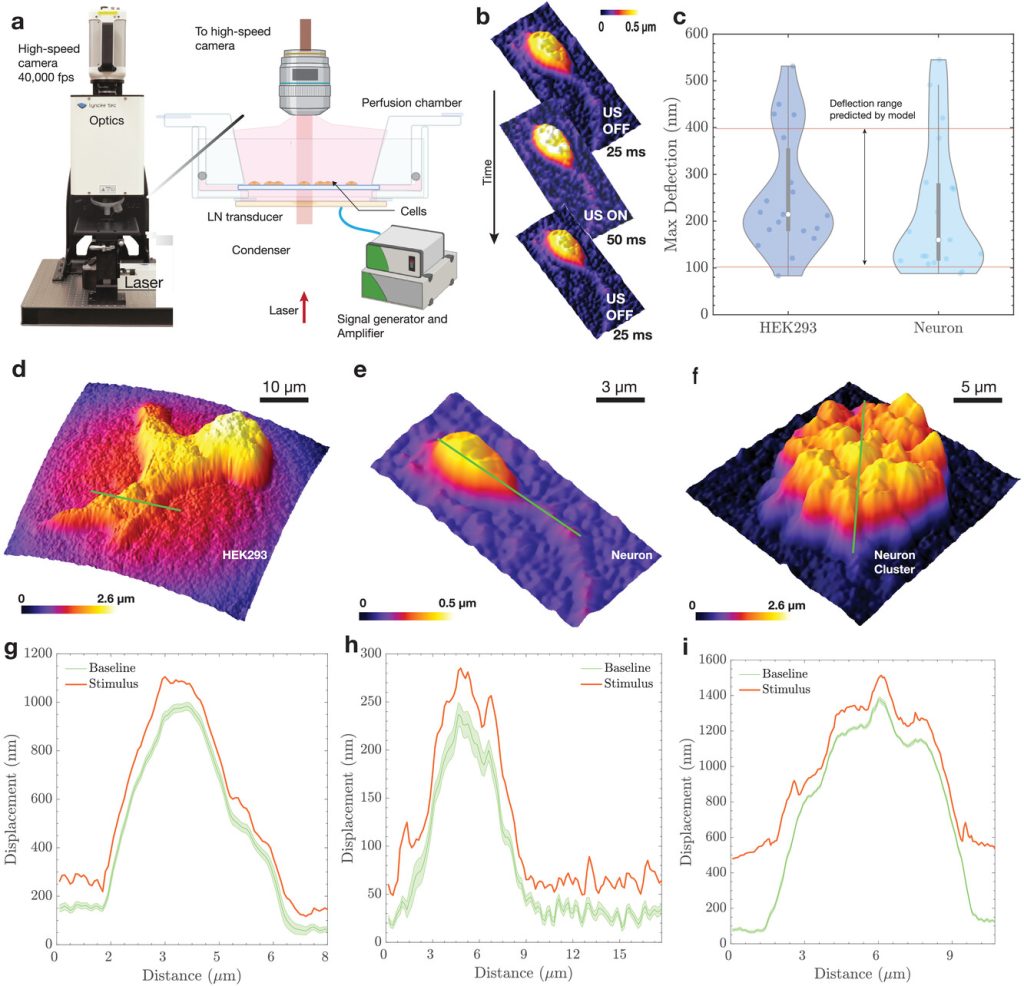
Mechanisms of Action. While the application of sonogenetics is rapidly expanding, understanding how cells sense mechanical waves is a core interest for our lab. We are probing how mechanical pressure waves are transduced into electrochemical signals in the brain. We recently used high-speed digital holographic microscopy to resolve cellular dynamics at kHz frequencies during stimulation.
- Nanoscale deflections: We demonstrated that cell membranes physically deflect by approximately 150 nm upon exposure to low-intensity ultrasound.
- Predictive Modeling: We developed a biophysical model linking acoustic pressure to membrane deflection and subsequent voltage changes.
- Model Validation: We validated our model using whole-cell patch-clamp validation, we proved that ultrasound-evoked deflection is likely sufficient to drive cellular depolarization.
We are investigating how transmembrane proteins sense these ultrasound-triggered mechanical forces at the molecular level. Furthermore, we are also exploring how the local lipid environment, including membrane stiffness and curvature, influences channel gating kinetics.
Beyond the Channel: Cellular Dynamics. To bridge the gap between single-channel gating and systemic biological effects, we are employing -omics approaches to profile the broader cellular response to ultrasound. This approach provides a holistic view of the signaling pathways activated during ultrasound and sonogenetic modulation. By mapping these high-resolution cellular responses, we ensure that our toolkit remains highly specific, minimizing off-target effects and provide a mechanistic understanding for non-invasive neuromodulation in complex tissues.
Resources & Collaborations. We are committed to accelerating the field of sonogenetics and welcome inquiries for collaboration and technical support regarding sonogenetic implementation. Please visit our sonogenetics.salk.edu for more information.
Neural mechanisms driving C. elegans behavior
We investigate how animals make strategic decisions in complex foraging contexts. How do animals integrate relevant external and internal parameters to plan foraging strategies that balance risk and reward? Which factors determine how animals value overall long-term success over short-term food rewards? Foraging strategies are critical for dealing with conditions in which feeding conflicts with another goal, such as exploration, predator avoidance, or territorial defense. However, little is known about how these foraging strategies are implemented. To answer these questions, we design naturalistic behavior paradigms that elicit rich and context-specific responses, build computational models of behavioral algorithms, and finely tune experimental parameters to conduct granular analyses. By studying nematodes with simple nervous systems and powerful genetic tools, we can link molecular and neural mechanisms to the decision processes that guide strategic foraging behaviors. Our work has revealed surprisingly sophisticated and advantageous strategic decision-making in nematodes with only ~300 neurons.
Credit: Amy Pribadi
Predator-prey interactions: The predatory nematode Pristionchus pacificus competes with the prey nematode Caenorhabditis elegans for bacterial food. We use this predator-prey system to study the predator’s response to the prey , as well as the prey’s response to the predator. We have demonstrated that P. pacificus can switch between predatory and territorial foraging strategies when both prey and bacterial food are present, a process mediated by octopamine (invertebrate homolog of norepinephrine). By using neuroeconomics and foraging theory to model the strategic value of biting, we found that P. pacificus is motivated by predation when it bites larval C. elegans (easy to kill, low competitive threat), and by territorial defense of bacterial food when it bites adult C. elegans (difficult to kill, high competitive threat). We also study how C. elegans adjusts its foraging strategy when it encounters both food and predators. While we have previously shown that C. elegans exhibits an escape response to sulfolipids in P. pacificus secretions, we are now interested in understanding how the experience of being bitten on a predator-inhabited bacterial food patch leads to changes in C. elegans behavior.
Neuronal signaling pathways: We have been probing the role of neurexin and other autism-associated genes in modifying C. elegans behavioral outputs. We discovered that neurexin mutants don’t join social aggregates while foraging for food. Using a combination of genetics, functional imaging and behavioral analysis we showed that this is behavior requires some, but not all neurexin isoforms and is driven by a change in the number of pre-synaptic sites at specific chemosensory neuron synapses. Moreover, these and other neurexin behavioral deficits are independent of neuroligin implying interactions with other post-synaptic receptors. We plan probing these neuronal signaling pathways with the goal of defining how information is altered at synapses and also provide a platform for developing diagnostic and therapeutic tools for neurological conditions.